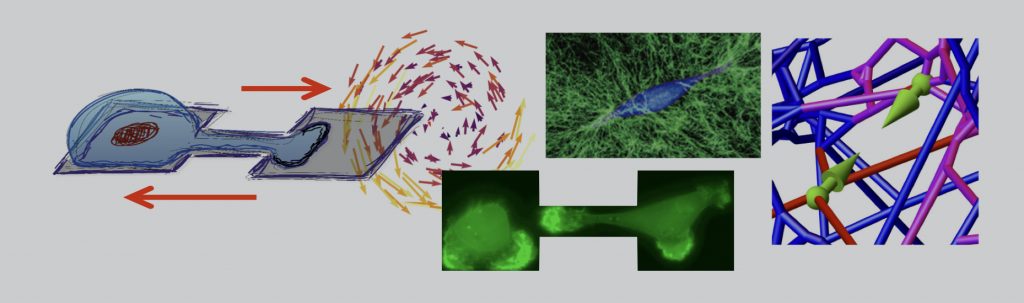
Cell and Tissue Dynamics
In all stages of life and death, from immune response and embryogenesis to cancer, migrating cells are key players. We are intrigued by how the mechanics and geometry of the environment of living cells impacts their migratory behavior. Cell motility is powered by a complex cytoskeletal machinery with numerous interacting molecular constituents that are subject to intrinsic noise. Nonetheless, at a larger scale, cells reliably perform vital motility functions. In physiological contexts, cells migrate effectively through highly structured and confining environments such as extracellular matrices, intravascular networks or epithelia. In all these settings, cells constantly interact with their mechanical micro-environment, and with one another. We develop physical approaches to uncover how these interactions determine the behavior and stochastic migratory dynamics of cells.
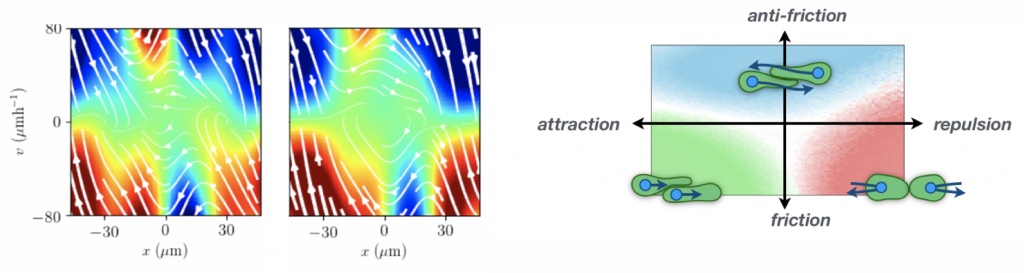
A key challenge is to determine a theoretical description for the emergent dynamics of confined cell migration and cell-cell interactions. The search for simple laws that underlie cell migration is complicated by the intrinsically variable nature of living cells. We develop theoretical frameworks to disentangle the deterministic and stochastic contributions to the observed dynamics. By analyzing stochastic trajectories of migrating cells, we delineate single cell behavior from how cells interact with other cells and their micro-environment. Put simply, we learn a stochastic equation of motion of confined and interacting cells directly from data. This approach offers a dynamical systems perspective, in which deterministic cell behaviors are characterized by properties of their nonlinear dynamics. Achieving a quantitative understanding of the stochastic migratory dynamics of cells at the behavioral level yields key insights into both the underlying molecular mechanisms and the biological functions associated to these behaviors.
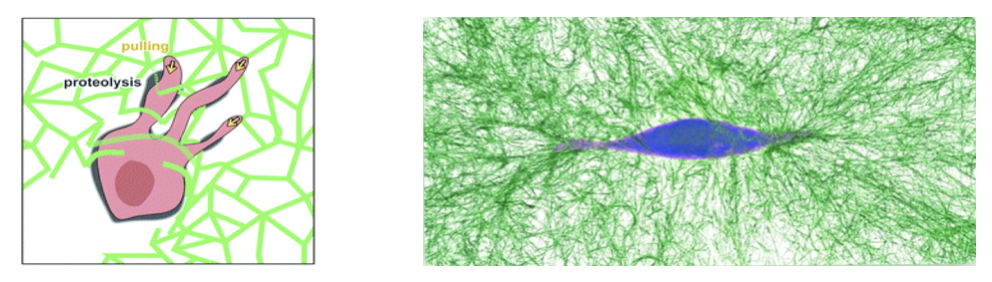
The behavior of living cells is influenced by the mechanics of the substrate on which they live and move. In tissues, living cells frequently interact mechanically with a 3D fibrous microenvironment: the extracellular matrix. It is still unclear what the network mechanics of this matrix looks like from the perspective of such a cell and how it affects cellular mechanosensing. To understand how the extreme mechanics of this matrix affects the behavior of embedded cells, we study how cell-generated forces propagate through the extracellular matrix and impact the mechanics of their 3D extracellular environment. With these approaches, we aim to uncover how cells interact with the extracellular matrix at the microscopic scale, to adapt to and control the mechanics of its surrounding tissue.
Further reading:
Learning the dynamics of cell-cell interactions in confined cell migration
David B. Brückner, Nicolas Arlt, Alexandra Fink, Pierre Ronceray, Joachim O. Rädler, Chase P. Broedersz
PNAS 118 (7), e2016602118 (2021) [arXiv:2008.03978] [LMU Press Release]
Stochastic nonlinear dynamics of confined cell migration in two-state systems
David B. Brückner, Alexandra Fink, Christoph Schreiber, Peter Röttgermann, Joachim O. Rädler, Chase P. Broedersz
Nature Physics 15, 595–601 (2019)
Cell contraction induces long-ranged stress stiffening in the extracellular matrix
Yu Long Han, Pierre Ronceray, Guoqiang Xu, Andrea Malandrino, Martin Lenz, Chase P. Broedersz+, Ming Guo+
PNAS 115 (16), 4075-4080 (2018) [arXiv:1709.00793]
Physical limits to biomechanical sensing in disordered fibre networks
Farzan Beroz, Louise M. Jawerth, Stefan Münster, David A. Weitz, Chase P. Broedersz, Ned S. Wingreen
Nat. Commun. 8, 16096 (2017) [arXiv:1608.01620]
Guiding 3D cell migration in deformed synthetic hydrogel microstructures
Miriam Dietrich, Hugo Le Roy, David B. Brückner, Hanna Engelke, Roman Zantl, Joachim O. Rädler, Chase P. Broedersz
Soft Matter 14, 2816-2826 (2018)
Fiber networks amplify active stress
Pierre Ronceray, Chase P. Broedersz, Martin Lenz
PNAS 113 (11), 2827–2832 (2016)